Months into the unfolding coronavirus crisis, and four weeks into writing this blog, new research results are giving context to how the virus arrived in the first place. SARS-CoV-2 is a zoonotic virus, meaning it first emerged in an animal and, through a chain of transmission, infected humans. Although we may never trace exactly how the virus reached humans in the first instance, what we are learning about COVID-19 infection is helping us begin to understand why it happened. This week’s post gives insight into recent research that delves into the basic biology of how the virus infects cells, and how cells respond when infected. These results are guiding promising therapeutic approaches, and will be vital resources in preparing for future outbreaks.

1. How do zoonotic viruses affect humans?
A survey of dozens of gene expression datasets from different tissues revealed that the most prominent cell type with high expression of genes associated with SARS-CoV-2 entry (such as the ACE2 receptor, discussed below) were nasal epithelial cells, which play a role in innate immunity. An independent analysis of those and other data found similar associations in susceptible cell types, between genes mediating viral entry and regulators of inflammatory programs.
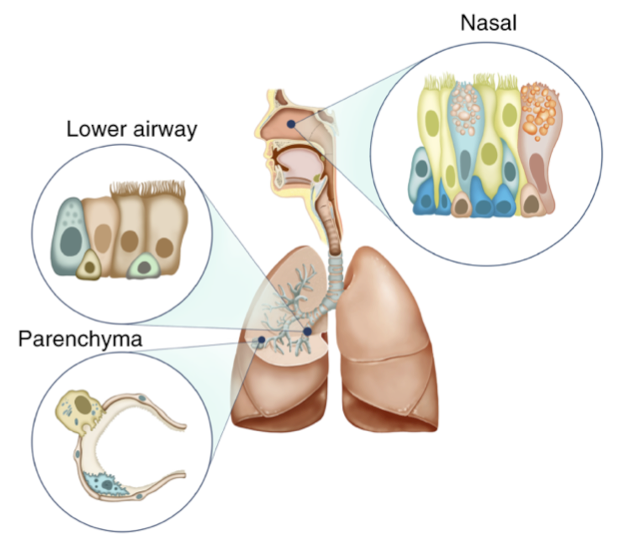
These studies suggest that when SARS-CoV-2 is infecting those cells that it can, the cells react by switching on an inflammatory response. Normally, inflammation is a signal to the immune system that something is awry, but an organ that is overloaded with virus produces too much signal, sending the immune system into a life threatening ‘cytokine storm’. Indeed, overactive cytokine release has been identified as a feature of severe COVID-19 infections.
Humans’ intense reaction to this zoonotic virus provokes an intriguing question: how was this virus ever tolerated in another species in the first place?
Early evidence of DNA sequences suggests Sars-CoV-2 may derive from a similar strain of bat coronavirus. This would not be the first time a bat coronavirus made the jump to humans, as the coronaviruses that cause SARS (2002–2004) and MERS (2012) both likely originated in bats. Why, then, do many of the most nefarious new infections derive from these reclusive, nocturnal creatures? One intriguing hypothesis begins by observing that bats are the only mammals that fly, and immensely taxing and physically stressful activity. If bats’ immune systems reacted to stress as intensely as humans’ they would constantly be flying themselves sick, so over the course of evolution they have suppressed their immune response. Now, when a new virus emerges in bats, it simply replicates, without hurting its host too much. Make the jump to humans, however, and the immune responses (and patient outcomes) are intensified.
One paper recently estimated that there were 1.6 million potentially zoonotic viruses, of which fewer than 1% have even been identified. Most zoonotic viruses simply aren’t equipped to make the jump from animals to humans, and the risk of any single virus’s leading to a pandemic is low. But as pandemic-prevention expert Peter Daszak puts it, “when you multiply a 10-million-to-one event by the total number of animal-human interactions, the probability isn’t that low after all.” The coronavirus pandemic, then, is a reminder of how deeply intertwined we humans are in the natural world. Differences in human and bat immune biology distinguish outcomes to the infection, but it is our biological similarities and ecological overlap that make the jump possible. The biological system common among mammals that allows SARS-CoV-2 to enter our cells is currently ‘under the microscope’ (so to speak) in the search for remedies, and is a target for some promising therapies that take a simple approach to a complex problem.
2. Block spike or ACE. No, it’s not volleyball.
Coronaviruses gain entry into cells by using a so-called ‘spike protein’ to recognize and bind a ‘receptor protein’, tricking the cell into engulfing it. When it meets our mammalian cells, SARS-CoV-2 recognizes the ACE2 receptor, featured prominently on the surface of cells in the lung and small intestine, among other tissues. The virus molecule that initiates this, SARS-CoV-2’s spike protein, is coated in glycosylations, tiny tree-branches of sugars that decorate the protein surface.

These modifications help hide the virus protein’s surface from our immune systems by making it hard to access, blocking our natural antibodies from recognizing their specific target. Glycosylations pose a similar problem for synthetic antibodies and small molecules developed for diagnostic and clinical goals, so a distinct strategy is needed that does not rely on directly detecting the virus’s spike protein. Rather than recognizing the virus protein directly, some studies are focusing on the ACE2 receptor as both a therapy and a therapeutic target.
One recent study reasoned that if the spike protein specifically recognizes cells’ ACE2 receptor, then perhaps flooding the infection with extra, free-floating ACE2 could capture most of the virus before it had the chance to infect a new cell. Using miniature model organs, called ‘organoids’, they found that indeed, ‘clinical grade’ ACE2 protein did reduce viral growth. A different study tried an opposite approach: rather than adding receptor to capture the spike protein, the authors suggested that dosing nicotine could ‘capture’ the cell-surface receptor, preventing the virus from binding and entering. Curiously, this hypothesis emerged from the observation that in both France and China, there was a lower proportion of smokers in patient cohorts (France: 5%, China: 12.6%) than in the general population (France: 35%, China: 26%).
While these potential therapies await clinical trials and approval, we can appreciate the detailed knowledge required to craft a reasoned intervention. Continuing to study the virus’s basic biology will reveal fresh details that will inform ongoing attempts at remedies, but such research is also the foundation of efforts to anticipate and preclude the next viral outbreak, such as CEPI, the Coalition for Epidemic Preparedness Innovations. Knowing where the virus came from, and how it came to us, are essential for managing the virus today, and preventing outbreaks in the future.
SARS-CoV-2 is smaller than the wavelength of visible light (~90nm vs. 380–740nm), so instead of regular pictures, scientists and artists have been sharing their individual and gorgeous visualization of the virus.
Science continues to supply guidance and insight despite the virus-imposed slow-down, and serves as a source of hope during otherwise uncertain days. While keeping up can seem overwhelming, Princeton GMOP is here to help bring you important updates on our understanding of the virus. What was your favorite highlight from this week? Did we miss anything that caught your attention? Comment on this article, and let us know at gmop@princeton.edu.
Ezra Levy, Noel Park, and Jeffrey Lee are graduate students in the Department of Molecular Biology at Princeton University.